One of the commonest questions asked by Creationists is “If evolution is real, why are there no transitional fossils?”
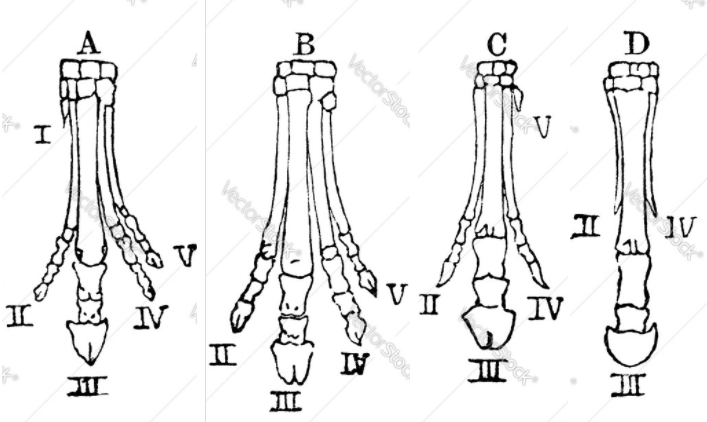
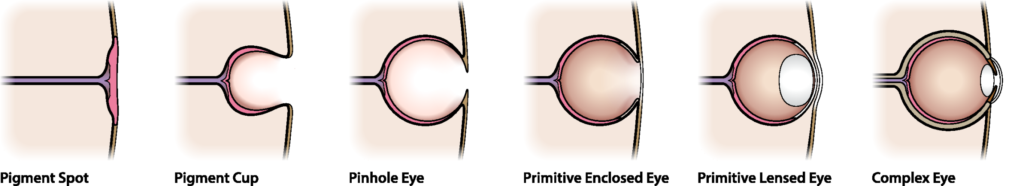
In fact, every fossil, every species, every individual, is in transition between what went before and what came after, except for those on the brink of extinction (and even they could be said to be in transition between what went before, and dead). You can line up fossils from bottom to top of the fossil record and see eyes develop from mere patches of light-sensitive cells to the complex eyes of a mammal, a spider, an octopus; the horse’s hoof go from four toes to one; the whale’s nostrils move from the end of the snout to the top of the head, all in small increments with many intermediate stages, all in the same order all around the world.
Of course every creature is complete as itself, otherwise it couldn’t live and reproduce, which is a necessary pre-condition for evolution. Creationists seem to think that we should find fossils of creatures with half a non-functional wing or eye, with natural selection somehow keeping them alive so that they could have a functional wing in 200,000 years. That of course would only be possible if Creationism were true.
The mechanism of natural selection, which is the main engine of evolution, is that family lines whose particular gene variants (alleles) best fit them to reproduce in their environment tend to have the highest number of surviving, fertile offspring, so that those particular gene variants become more common from generation to generation, alterring the average form of the population. Between close relatives genes are fungible, so an individual within a family line may pass their genes on directly, by having their own offspring, or may do so by promoting the welfare of the offspring of close kin, with whom they share many alleles. In terms of passing on your genes, two nieces or nephews are as good as one direct child.
Mutations which result in new alleles whose effects are neutral may also survive by chance. Any mutation which is damaging or has significant cost will be selectively bred out of the population, as lines with that allele have fewer surviving fertile offspring, unless it is masked by other genes which override it, or by other alleles of the same gene to which it is very recessive; or if it happens to be positioned very close on its chromosome to a very beneficial gene which is being strongly selected for. In the last case the harmful variant may piggy-back on its beneficial neighbour until the chromosome happens to split in the small space between them, separating them so that selection pressure can act on them individually.
There are also some alleles which survive because although they have a significantly harmful effect, they have an even more significant benefit, resulting in a net gain. The classic example is sickle cell anaemia. If you have two copies of it, one from each parent, you will have a crippling blood disorder: but if you have just one copy you will be healthy and also resistant to malaria. If each parent has one copy, on average half their children will be resistant to malaria, a quarter will be cripplingly ill and a quarter will be neither ill nor resistant, so in areas affected by malaria the benefits of this allele to a family line outweigh the costs.
So, every stage in an evolutionary sequence has to be beneficial, or at least not so harmful that selection pressure would weed it out. Even the most primitive eye-spot conveys the ability to tell light from dark; even the most rudimentary wing gives a little lift when running, or turns a potentially fatal fall from a tree into a parachute jump or a glide; and then selection builds on that in successive tiny increments, each slightly more effective in its particular environment than what went before. Every organism functions as itself, but its offspring will be very slightly different, and their offspring slightly different still, and after ten thousand generations you have a different organism.
Take any two points in a line of descent and there will be a mid-point, and where we have a lot of fossils for a sequence of organisms, we can see it. Sometimes the sequence will be smooth, and sometimes, if the environment changed suddenly, you will find the changes speeding up and then slowing down again once the environment stabilises (called punctuated equilibrium), but you can still track those changes. Gary Meaney on Quora has a very nice sequence showing progressive fossils from the first chordates to humans. Of course there are many thousands more species which fit in between the examples in this sequence, but Gary’s answer shows selected highlights.

There is also this lovely sequence of fossil snails, showing a smooth transition from one species to another. I haven’t been able to find out what the species involved were (if I do I’ll update this essay to name them), but again, these six are just selected highlights, reported to have been picked from a group of over 15,000 individual fossils which fitted between the one at far left and the one at far right. Take any point in a vertical column of these shells, stacked up over the millenia, and the fossils an inch or two above or below it will be so similar as to be indistinguishable but the fossils a foot or two above or below it will be clearly different, forming a sequence from one form to the next.

We can see similar smooth sequences occurring horizontally with living species, rather than vertically through time, with what are called ring species. What that means is that you get a series of closely related but slightly different groups spread out along a sequence, in such a way that Group A can breed with B, B can breed with C, C can breed with D, D can breed with E, but A and E, if they meet, cannot interbreed and are clearly different species, and if any of the intermediate groups were to go extinct there would now be two totally separate breeding populations with no cross-link. The classic example is the Larus gulls, which form a ring of small populations around the Northern Hemisphere. The two ends of the sequence, the herring gull Larus argentatus and the very similar but darker lesser black-backed gull L. fuscus, are not interfertile but they are connected by a smooth sequence of variation through small populations.
Creationists will never be satisfied with any sequence, however complete, unless we can show fossils of every single generation of every single line of descent. Fill in one big gap between two fossils and you create two smaller gaps for them to scream about. But one of the beauties of understanding natural selection is that when we see a large gap we can predict what we would expect to find filling that gap, and then go out and find it.

One of the nicest recent examples is Tiktaalik roseae, first discovered on Ellesmore Island in 2004. We knew from their skeletal anatomy, and the fact that some of them can breathe air, that lobe-finned fish were probably ancestral to tetrapods – four-legged terrestrial vertebrates and their descendants (even though a few of those descendants have since lost their limbs and/or gone back to the sea). Initially there was quite a big gap in the fossil record between lobe-finned fish and tetrapods, but we knew there should be an intermediate form, and “Seek and ye shall find”. We sought, and we found Tiktaalik, the fish that was still a fish but also very nearly a newt, with muscular, weight-bearing forelegs with actual wrists so the fins could be rotated partially forwards and used as proper feet; with primitive nostrils and a sturdy ribcage, suggesting it had lungs as well as gills; and with a flexible neck instead of the bony gill-plates which hold the necks of most fish rigid.
Another extremely nice example was announced very recently, in March 2022. Fossils of Coleoid cephalopods (squid, cuttlefish and octopodes) are hard to find because they have few hard parts – just beaks, and a minimalist internal supporting strut in squid and cuttlefish – and soft-bdoed animals are only preserved as fossils in rare, very fine-grained shale or similar. Squid and cuttlefish each have eight arms (that is, shortish limbs with suckers along their length) and two tentacles (long, smooth limbs with suckers only on a pad at the tip, used to shoot out and grab prey). Octopodes just have eight arms, no tentacles. But there exists an intermediate animal which is called a vampire squid, although it is closer to octopodes.

A vampire squid has eight arms with nasty-looking spikes on the inner surface, joined almost down to the tips by webbing which makes the animal look like a psycho umbrella, and swims by means of two flappy little fins. They can actually evert the umbrella part and fold it back to protect their body, which is now protected by bristling rows of spikes (they are apparently fleshy rather than sharp, but they look the part). But they also have two long, very thin, retractile “velar filaments” which they trail through the water, using them to sense ocean currents and proximity to anything edible.
In March 2022 it was announced that a 328-million-year-old fossil cephalopod, which had been dug up in Montana in 1988 but had not been properly examined until now, was a new species of vampyropod, a close relative of vampire squid and of octopodes. The new animal, christened Syllipsimopodi bideni in honour of Joe Biden, was mostly vampire-squid-like in its anatomy but its body was bullet-shaped, more like a regular squid, and it had eight arms plus two tentacles like a squid’s, but very thin. These intermediate tentacles make it pretty definite that vampire squid started as regular squid and then slowly lost their tentacles, on the way to becoming octopodes.
Going back a bit, scientists had long suspected that ants were descended from wasps, and in 1966 they discovered an intermediate form, Sphecomyrma.
We don’t find an intermediate form every time we look for one, of course, because fossilisation is a rare event and finding the fossils later even rarer. But we find them often enough that for Creationists to scream that the existence of a few gaps disproves the whole edifice is like somebody claiming that books aren’t a real Thing because they found a copy of War and Peace with seven random pages missing.
For all these transitional forms to be the result of direct creation by a deity, rather than evolution, you would have to assume that the Creator came back every few hundred years for millions upon millions of years, wiped out a given line of organisms and then remade them slightly more like their modern forms. For Young Earth Creationism to be true, with the world only 6,000 years old and a single week of creation, you’d have to assume that the Creator created millions upon millions upon millions of species, then killed nearly all of them, then stacked the bodies up vertically by order of how similar they were to the few surviving species, with the most similar at the top, arranged in such a way that they formed branching trees of similarity, in the same order all round the world, with the lowest levels occupied only by microorganisms, followed by a smoothish progression from simple worm-like creatures all the way up to modern, complex life
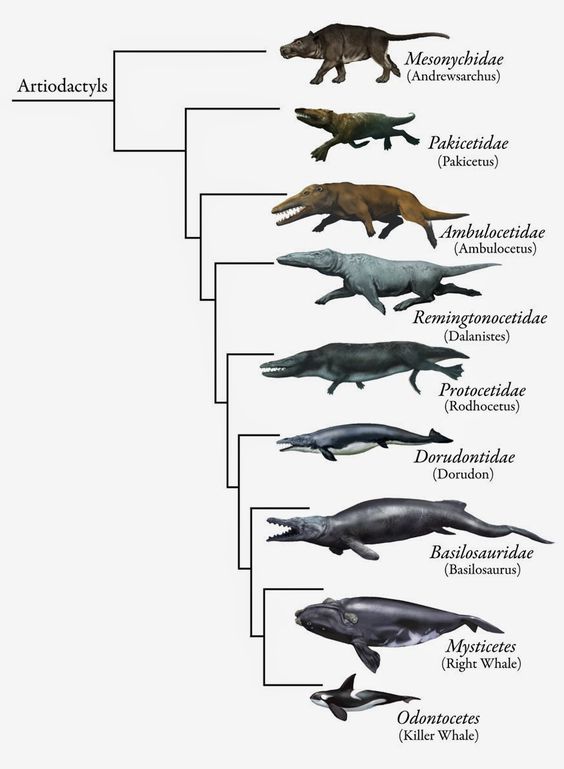
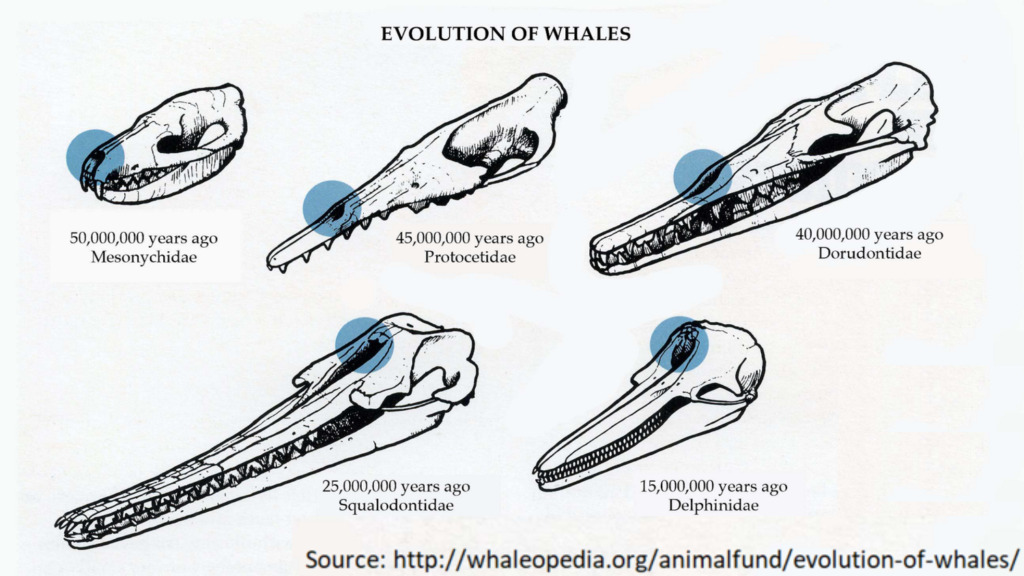
Even more remarkably, it created and arranged them with the similarites all moving in the same direction. That is, if you look up and down the tree of forms that are most like surviving whales, for example, the movement of the nostrils from the end of the nose up to the forehead, the change from forefeet to flippers, the reduction of the hind legs and the development of a tail fluke all move in the same direction, beginning with a hoofed land animal and gradually becoming more whale-like. If you line them up by nostril position, you’ve also lined up all the other features in a smooth progression: you don’t find the nostrils forming a smooth sequence while hind legs and tail flukes appear and disappear, grow and shrink at random.
Then that hypothetical Creator interleaved these stacks of millions of dead bodies with evidence of long lives lived which according to Young Earth Creationists they didn’t get to live – babies or food in their bellies; scars from old, healed injuries on their bodies; footprints; eggs both hatched and unhatched; dung; whole ecosystems of plants mingled with the animals.
Some Creationists argue that yes, that’s exactly what God did – He made the fossil record especially to deceive us, in order to test our faith. So, the argument is:
- The Creation story in Genesis must be literally true, because the Bible is the word of God and God doesn’t lie (or, apparently, know how to use metaphor or simile, so we must conclude that the breasts of the girl in the Song of Solomon were actual baby deer, with tiny hooves).
- The fossil record disagrees with the story in Genesis, so God must have created fossils in order to lie to us, because Genesis must be literally true because God wouldn’t lie to us.

Of course, many Creationists who claim that there are no transitional fossils, don’t even understand what a transitional fossil is. They imagine that if evolution were true, we would be able to find forms that are intermediate between any two current organisms, in a very crude way, such as a duck with a crocodile’s head, or a rabbit with eagle’s wings. In fact, while such forms would be perfectly possible in a Created world, and may some day be possible in the laboratory, they are impossible to achieve by natural evolution. That such forms do not exist is one of the best proofs of evolution. [And please, nobody say “duck-billed platypus” – that may look like a bird’s beak, but it’s actually a specialised lip, used to detect electrical signals under water.]
New species occasionally form in a single generation by a single major chromosome mutation (the rearrangement, duplication or loss of parts of chromosomes, of whole chromosomes or even of the whole genome), or by a chance hybridisation. Normally, however, species form gradually by natural selection – although just how gradually depends on the strength of the selection pressure to which they are exposed, the amount of variation already present in the species, the length of a generation and how many offspring are in each generation.
Speciation may be anagenic, where a single population changes over time until it becomes distinctly different from its ancestors, or cladogenic, where a population becomes completely split into two or more groups which are subject to different selection pressures and are no longer able to mingle and pool their genes, so that they head off in different directions. Cladogenic speciation may be allopatric, where two roughly equal groups of the initial population become separated by a physical barrier which prevents interbreeding even before they speciate; peripatric, which is the same as allopatric speciation except that you have a main group and then one or more much smaller groups splitting off around the edges; parapatric, where the separated groups still have some contact and a small amount of gene-sharing at the edges of their ranges; and sympatric, where a new species arises spontaneously within a population of an existing species with which it is still in contact and with whom it can initially still interbreed – this one is controversial, but we can see it beginning to happen within populations of the ship rat, Rattus rattus, in India, where chromosome mutations have resulted in three or four seprate groups who can still breed with each other, but do so with reduced fertility which will probably cause them to drift further apart in the future.
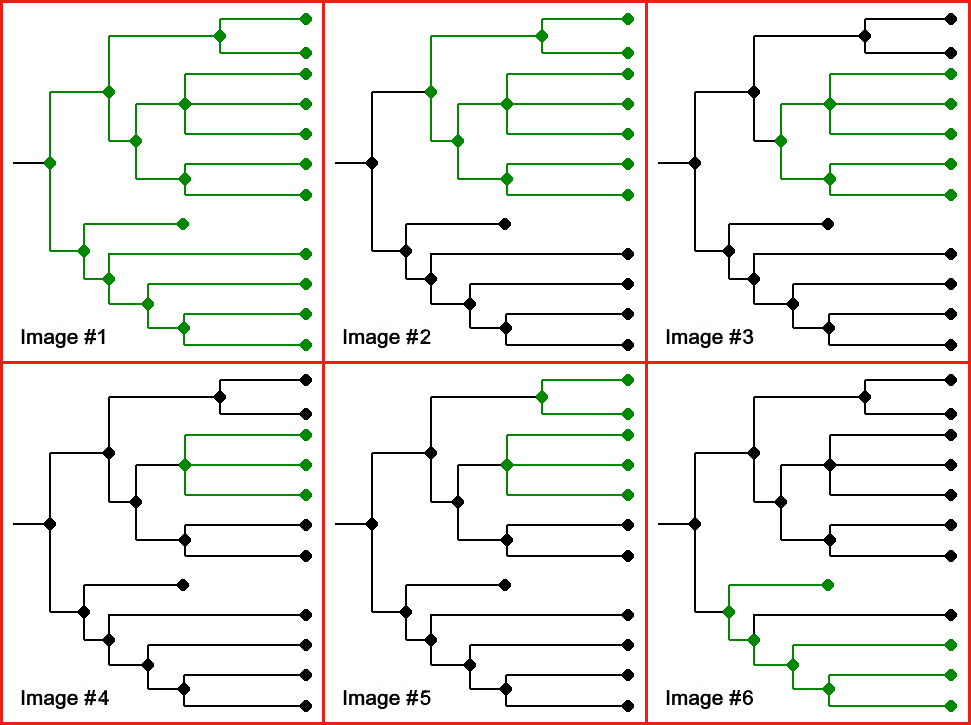
These splitting points form what we call clades, with each clade consisting of a base species and then all the species which descend from it, and none that don’t. If each node in the accompanying charts is a species, joined by lines of descent, then the green areas of Image #1 form a clade and so do the ones in Images #2-#4, but the ones in Image #5 show a polyphyletic group (two or more separate clades not extending back to their common ancestor) and Image #6 shows a paraphyletic group (all the species involved share a common ancestor, but there are other descendants of that ancestor who are not included).
Reality is always messier than human classification systems, and precisely because species form slowly, by evolution, there is often a stage where cladogenic, diverging species are distinctly different in form but are still able to interbreed if they happen to meet, which can make the starting point of a clade hard to pinpoint. Nevertheless, these branching points are real. Think of a river that splits into two, but for the first couple of miles there are streams and marshy bits which still link the two sides, after which they become wholly separate.
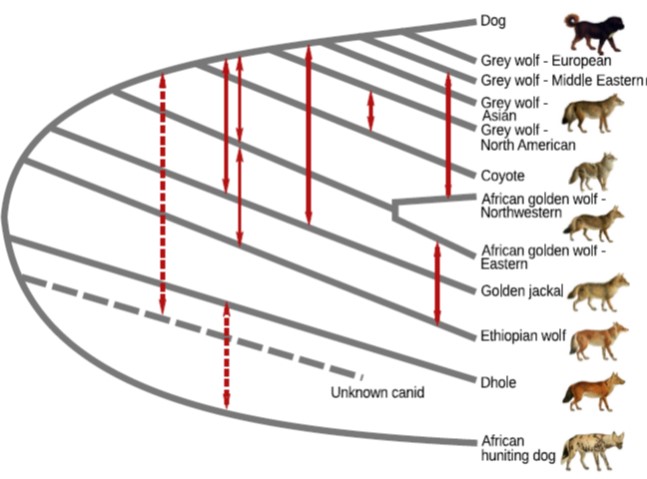
Here you can see that members of the dog family tend to hybridise, but that the dhole/painted dog group hasn’t interbred with the true dog/wolf group since not long after they separated (and then only via an unknown canid).
Over time the arrangement of genes, and eventually even the physical body, of the two species becomes too different for them to be able to have fertile offpsring together. This can happen very rapidly, or it can take an astonishingly long time. In 2019 a laboratory in Hungary accidentally created a “sturddlefish”, a hybrid of the American paddlefish (Polyodon spathula) and the Russian sturgeon (Acipenser gueldenstaedtii), despite the two species having separated some 184 million years ago. I don’t think we know yet whether these hybrids will be fertile as they are still too young to tell. They probably won’t be, as only a few of the initial hybrid embryos survived:, but the mere fact that any of them were viable is remarkable enough.
Once two lines have separated completely and can no longer interbreed, however, they can no longer mingle their genes (except occasionally when a virus picks up a few genes from the genome of one species and carries them to another). Any features which evolve in a given line after a complete splitting point cannot be shared with the species on the other side of the split. Even the earliest attempts at a rabbit’s furry rabittyness and an eagle’s wings evolved about 150 million years after the lines leading to mammals and birds split. A rabbit and an eagle are far too different, physically and genetically, to be able to interbreed, and the genes for the eagle’s wings cannot somehow be passed backwards through its ancestors for 300+ million years to its last common ancestor with mammals, then forwards again to rabbits.
In the same way, if you come from an all-blond family and you marry a partner with black hair, your children may then have black hair, but the gene for black hair will not be passed from your child to you, and then to your parents, and then to your grandparents, and then back down to your aunt, and then to your cousin, causing your cousin to also have a black-haired child. Genes which are added into a line can only be passed forwards, not back.
In order for a species genuinely to combine the characteristics of two other species, it either needs to be a hybrid (which would only normally happen if the two species split very recently); or have evolved those characteristics independently by convergent evolution; or it has to have a long line leading back to a common ancestor with those species, which split from them after those characteristics evolved. If the last common ancestor of crocodiles and ducks had itself had both feathered wings and a crocodile’s jaw, then you could get three lines arising from that common ancestor, one of which kept both wings, feathers and jaw; one of which kept the wings and feathers but lost the jaw; and one of which kept the jaw and lost the wings and feathers. But we know from the fossil record that feathered wings arose after the ancestor of birds parted company from crocodiles.
So, again, a Creator God could certainly create a croco-duck which genuinely combined the characteristics of two current organisms without a long history going back to when those organisms split, and with luck and a following wind a scientist might be able to do so, but evolution cannot. A Creator God could make an organism with half-formed, useless features, but evolution cannot. That we do not find such forms is not a weakness in the theory of evolution, as Creationists imagine, but one of the strongest evidences for it.

Fascinating
Thank you